Recent research
<日本語/English>
 Spin-crossover complexes are well known as complex molecules that can switch their electronic states in response to external stimuli, and have been studied intensively in our laboratory. On the other hand, multinuclear metal complexes bridged by cyanide ions can exhibit Electron-Transfer-Coupled Spin Transition (ETCST), in which electron transfer between metal ions coupled with spin transition occur in concert in response to external stimuli. Although electron transfer and hydrogen bond formation are closely related in biological systems, the search for material systems in which the two are strongly correlated in molecular solids has not yet been sufficient.
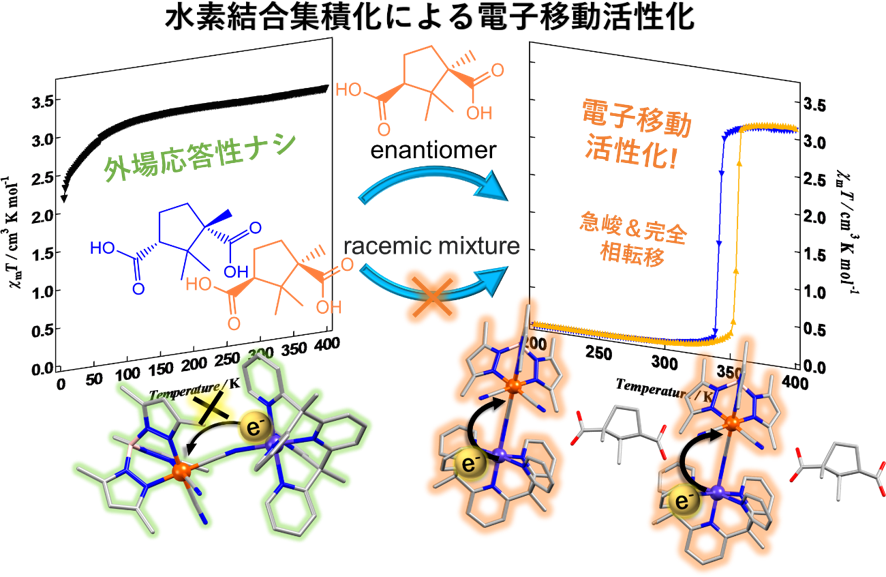
In this study, we focused on the [CoFe] dinuclear complex, which is the smallest unit of Prussian blue analogues, and clarified the effect of hydrogen-bonding assembly into the electron transfer behavior. The CoFe dinuclear complexes with different ligands, [CoFe(CN)3(tp*)(PY5Me2)](ClO4) (1) and [CoFe(CN)3(tpMe)(PY5Me2)](ClO4) (2) (tp* = hydrotris(3,5-dimethylpyrazol-1-yl) tpMe = hydrotris(3-methylpyrazol-1-yl)borate, and PY5Me2 = 2,6-bis(1,1-bis(2-pyridyl)ethyl)pyridine) were synthesized. The temperature-dependent magnetic susceptibility measurements of 1 and 2 showed that none of them exhibited ETCST as the temperature changed. The reaction solutions of 1 and 2 with (+)-camphoric acid as a chiral carboxylic acid resulted in the formation of 3+ and 4+, which consisted of one-dimensional hydrogen-bonded assemblies. The magnetic behavior of the 3+ and 4+ was measured and a steep change was observed, indicating that it is possible to design 3+ and 4+ that are responsive to external fields by hydrogen bonding 1 and 2, which are not responsive to external fields. Furthermore, when the carboxylic acid used for integration was changed to a racemic form, only 4rac changed to a gradual transition behavior due to defects in the crystal lattice, indicating that the transition behavior differs depending on the construction unit and assembling method.
Spin-crossover complexes are well known as complex molecules that can switch their electronic states in response to external stimuli, and have been studied intensively in our laboratory. On the other hand, multinuclear metal complexes bridged by cyanide ions can exhibit Electron-Transfer-Coupled Spin Transition (ETCST), in which electron transfer between metal ions coupled with spin transition occur in concert in response to external stimuli. Although electron transfer and hydrogen bond formation are closely related in biological systems, the search for material systems in which the two are strongly correlated in molecular solids has not yet been sufficient.
In this study, we focused on the [CoFe] dinuclear complex, which is the smallest unit of Prussian blue analogues, and clarified the effect of hydrogen-bonding assembly into the electron transfer behavior. The CoFe dinuclear complexes with different ligands, [CoFe(CN)3(tp*)(PY5Me2)](ClO4) (1) and [CoFe(CN)3(tpMe)(PY5Me2)](ClO4) (2) (tp* = hydrotris(3,5-dimethylpyrazol-1-yl) tpMe = hydrotris(3-methylpyrazol-1-yl)borate, and PY5Me2 = 2,6-bis(1,1-bis(2-pyridyl)ethyl)pyridine) were synthesized. The temperature-dependent magnetic susceptibility measurements of 1 and 2 showed that none of them exhibited ETCST as the temperature changed. The reaction solutions of 1 and 2 with (+)-camphoric acid as a chiral carboxylic acid resulted in the formation of 3+ and 4+, which consisted of one-dimensional hydrogen-bonded assemblies. The magnetic behavior of the 3+ and 4+ was measured and a steep change was observed, indicating that it is possible to design 3+ and 4+ that are responsive to external fields by hydrogen bonding 1 and 2, which are not responsive to external fields. Furthermore, when the carboxylic acid used for integration was changed to a racemic form, only 4rac changed to a gradual transition behavior due to defects in the crystal lattice, indicating that the transition behavior differs depending on the construction unit and assembling method.
○ J. Am. Chem. Soc,
2024, 146, 35, 24238–24243.
 Ferroelectric liquid crystals (FLCs) are fascinating functional materials that have a remnant and electrically invertible polarization. To date, typical FLCs have been mainly realized by molecular design such as the incorporation of chirality into a given molecular structure. Here, we report for the first time ferroelectricity induced by spin transition via low spin ↔ high spin state associated with a crystal – liquid crystal phase transition in achiral molecules. These findings will undoubtedly lead to new strategies for the design of new FLCs based on metal-centered spin transitions.
Ferroelectric liquid crystals (FLCs) are fascinating functional materials that have a remnant and electrically invertible polarization. To date, typical FLCs have been mainly realized by molecular design such as the incorporation of chirality into a given molecular structure. Here, we report for the first time ferroelectricity induced by spin transition via low spin ↔ high spin state associated with a crystal – liquid crystal phase transition in achiral molecules. These findings will undoubtedly lead to new strategies for the design of new FLCs based on metal-centered spin transitions.

(a)Single crystal structure of [Fe(3C16-bzimpy)2](BF4)2
(b)Ferroelectric properties driven by SCO phenomenon accompanying crystal-liquid crystal phase-transition
○ Chem. Sci.,
2019, 10, 5843-5848.
 Luminescent Zn(II) clusters [Zn4L4(µ3-OMe)2X2] (X = SCN (1), Cl (2), Br (3)) and [Zn7L6(µ3-OMe)2(µ3-OH)4]Y2 (Y = I- (4), ClO4- (5)) exhibiting blue fluorescence at room temperature (λmax = 416~429 nm, Φem = 0.09-0.36) have been synthesised and investigated in detail. In one case the external heavy-atom effect (EHE) arising the presence of iodide counter anions yielded phosphorescence with a long emission lifetime (λmax =520 nm, τ = 95.3 ms) at 77 K. Single-crystal X-ray structural analysis and time-dependent density-functional theory (TD-DFT) calculations revealed that their emission origin was attributed to the fluorescence from the singlet ligand-centered (LC) excited state, and the phosphorescence observed in 4 was caused by the EHE of counter anions having strong CH-I interactions. To the best of our knowledge, this is the first observation of long phosphorescence involving the EHE arising from counter anions in luminescent Zn(II) clusters.
Luminescent Zn(II) clusters [Zn4L4(µ3-OMe)2X2] (X = SCN (1), Cl (2), Br (3)) and [Zn7L6(µ3-OMe)2(µ3-OH)4]Y2 (Y = I- (4), ClO4- (5)) exhibiting blue fluorescence at room temperature (λmax = 416~429 nm, Φem = 0.09-0.36) have been synthesised and investigated in detail. In one case the external heavy-atom effect (EHE) arising the presence of iodide counter anions yielded phosphorescence with a long emission lifetime (λmax =520 nm, τ = 95.3 ms) at 77 K. Single-crystal X-ray structural analysis and time-dependent density-functional theory (TD-DFT) calculations revealed that their emission origin was attributed to the fluorescence from the singlet ligand-centered (LC) excited state, and the phosphorescence observed in 4 was caused by the EHE of counter anions having strong CH-I interactions. To the best of our knowledge, this is the first observation of long phosphorescence involving the EHE arising from counter anions in luminescent Zn(II) clusters.

(a) Crystal structute of Zn(II) clusters
(b)Emission spectra of [Zn7L6(μ3-OMe)2(μ3-OH)4]I2 at 77K
○ Chem. Eur. J.,
2019, 25, 1-6.
 We have synthesized magnetic heptanuclear clusters of type [Ni7-xMx(HL)6(μ3-OMe)4(μ3-OH)2]Cl2 (M = Zn2+, Co2+, Mn2+). We have probed the local positions of Zn2+ ions by analyzing the magnetic interactions of these clusters in details. Such local positions of metal ions in dimetallic clusters incorporating metal ions bearing the same oxidation number are difficult to determine by X-ray diffraction. Furthermore, we have demonstrated that the Ni4Co3 cluster shows slow magnetic relaxation phenomenon, leading to a SMM based on the magnetic anisotropies of Co2+ ions. The present work demonstrates that control of the magnetic properties of heterometallic clusters can be achieved by changing the combination of metals present and provides a useful strategy which will promote both the better understanding and the future development of new magnetic clusters.
We have synthesized magnetic heptanuclear clusters of type [Ni7-xMx(HL)6(μ3-OMe)4(μ3-OH)2]Cl2 (M = Zn2+, Co2+, Mn2+). We have probed the local positions of Zn2+ ions by analyzing the magnetic interactions of these clusters in details. Such local positions of metal ions in dimetallic clusters incorporating metal ions bearing the same oxidation number are difficult to determine by X-ray diffraction. Furthermore, we have demonstrated that the Ni4Co3 cluster shows slow magnetic relaxation phenomenon, leading to a SMM based on the magnetic anisotropies of Co2+ ions. The present work demonstrates that control of the magnetic properties of heterometallic clusters can be achieved by changing the combination of metals present and provides a useful strategy which will promote both the better understanding and the future development of new magnetic clusters.

○ Dalton Trans.,
2018, 47, 16422-16428.
 The cobalt(II) complex [Co(Naph-C2-terpy)2](BF2)2·2MeOH (1·2MeOH) showed single crystal to single crystal transition, resulted in its desolvated product of 1. Complex 1 incorporating a highly distorted [CoN6] core was found to exhibit abrupt spin transition behavior, unlike 1·2MeOH, Q4 which showed gradual spin crossover behavior.
The cobalt(II) complex [Co(Naph-C2-terpy)2](BF2)2·2MeOH (1·2MeOH) showed single crystal to single crystal transition, resulted in its desolvated product of 1. Complex 1 incorporating a highly distorted [CoN6] core was found to exhibit abrupt spin transition behavior, unlike 1·2MeOH, Q4 which showed gradual spin crossover behavior.

○ Dalton Trans.,
2018, 47, 13809-13814.
 The dinuclear iron(III) complex [Fe(salten)2(bipytz)](BPh4)2· EtOH (1) was synthesized and its post-synthesis modification yielded [Fe(salten)2(bipydz)](BPh4)2·EtOH (2). The crystal
structures and magnetic behaviors of 1 and 2 were investigated by variable temperature XRD, magnetic susceptibility, and Mössbauer spectra measurements, with each of these dinuclear iron(III) complexes exhibiting 11 gradual spin crossover behavior.
The dinuclear iron(III) complex [Fe(salten)2(bipytz)](BPh4)2· EtOH (1) was synthesized and its post-synthesis modification yielded [Fe(salten)2(bipydz)](BPh4)2·EtOH (2). The crystal
structures and magnetic behaviors of 1 and 2 were investigated by variable temperature XRD, magnetic susceptibility, and Mössbauer spectra measurements, with each of these dinuclear iron(III) complexes exhibiting 11 gradual spin crossover behavior.

(a)Crystal structure of [Fe2(salten)2(Bipytz)]2+
(b)Postsynthetic modification
○ Z. Anorg. Allg. Chem.,
2018, 644, 729-734.
 Zinc(II) and platinum(II) complexes [M(X-4-C18-salmmen)] (M = Zn (1) and Pt (2), X = R, S (optical isomer) and rac (racemate), salmmen = N,N’-monomethylenebis-salicylideneimine) were synthesized
and investigated. The series of compounds given by 1 (R, S and rac forms) display liquid crystalline state behavior at 413 K, and exhibit
both ferroelectric and fluorescence properties. While the compounds given
by 2 (R, S and rac forms) do not display liquid crystal behavior but do exhibit ferroelectric and fluorescence properties. The ferroelectric behavior was confirmed by second harmonic generation (SHG) experiments. It is considered that the motion and fluctuation of the long alkyl chains in these complexes results in the observed ferroelectric properties.
Zinc(II) and platinum(II) complexes [M(X-4-C18-salmmen)] (M = Zn (1) and Pt (2), X = R, S (optical isomer) and rac (racemate), salmmen = N,N’-monomethylenebis-salicylideneimine) were synthesized
and investigated. The series of compounds given by 1 (R, S and rac forms) display liquid crystalline state behavior at 413 K, and exhibit
both ferroelectric and fluorescence properties. While the compounds given
by 2 (R, S and rac forms) do not display liquid crystal behavior but do exhibit ferroelectric and fluorescence properties. The ferroelectric behavior was confirmed by second harmonic generation (SHG) experiments. It is considered that the motion and fluctuation of the long alkyl chains in these complexes results in the observed ferroelectric properties.

(a) Structures of [M(X-4-C18-salmmen)] (M = Zn (1) and Pt (2), X = R, S and rac).
(b) Temperature-dependent P- E hysteresis curves for 1-rac.
○ Dalton Trans.,
2018, 47, 14288-14292.
 A sextuply linked capsule incorporating two Ni(II) cubane units is formed when Ni(II) salts are reacted with N-aminoalkyl salicylamidato ligands in a 4:3 ratio in methanol. With a short (propyl) alkyl substituent, additional chloride counter ions are included in the cavity by occupation of one apex of each of the linked cubanes, while with a longer (pentyl) substituent, perchlorate and tetrafluoroborate anions are included, probably by H-bonding involving hydroxyl groups on the inner apices of the cubanes, indicating that the cavity can be regarded as suited to binding of hydrophilic units despite the lipophilic character of its links. The dicubane capsules show ferromagnetic behavior typical of Ni(II) cubanes in general, with coupling constants dependent upon the nature of the salt-derived counter anions.
A sextuply linked capsule incorporating two Ni(II) cubane units is formed when Ni(II) salts are reacted with N-aminoalkyl salicylamidato ligands in a 4:3 ratio in methanol. With a short (propyl) alkyl substituent, additional chloride counter ions are included in the cavity by occupation of one apex of each of the linked cubanes, while with a longer (pentyl) substituent, perchlorate and tetrafluoroborate anions are included, probably by H-bonding involving hydroxyl groups on the inner apices of the cubanes, indicating that the cavity can be regarded as suited to binding of hydrophilic units despite the lipophilic character of its links. The dicubane capsules show ferromagnetic behavior typical of Ni(II) cubanes in general, with coupling constants dependent upon the nature of the salt-derived counter anions.

○ Dalton Trans.,
2018, 47, 9575-9578.
 Iron(III) complexes with an aromatic counteranion, [Fe(qsal)2]BS·MeOH·H2O (1), [Fe(qsal)2](NS)·MeOH (2), [Fe(qnal)2](NS) (3), and [Fe(qnal)2]PS·MeOH·CH2Cl2 (4) (Hqsal, N-(8-quinolinyl)salicylaldimine; Hqnal, N-(8-quinolinyl)-2-hydroxy-1-naphthaldimine, BS, benzenesulfonate; NS, 1-naphthalenesulfonate; PS, 1-pyrenesulfonate), show spin-crossover behavior, and the LIESST effect was observed in 1, 3, and 4. For 1, irradiation (808 nm) of the LS form at 5 K produced 59% conversion to the HS form, equal to the highest conversion known in such systems.
Iron(III) complexes with an aromatic counteranion, [Fe(qsal)2]BS·MeOH·H2O (1), [Fe(qsal)2](NS)·MeOH (2), [Fe(qnal)2](NS) (3), and [Fe(qnal)2]PS·MeOH·CH2Cl2 (4) (Hqsal, N-(8-quinolinyl)salicylaldimine; Hqnal, N-(8-quinolinyl)-2-hydroxy-1-naphthaldimine, BS, benzenesulfonate; NS, 1-naphthalenesulfonate; PS, 1-pyrenesulfonate), show spin-crossover behavior, and the LIESST effect was observed in 1, 3, and 4. For 1, irradiation (808 nm) of the LS form at 5 K produced 59% conversion to the HS form, equal to the highest conversion known in such systems.

(a) Crystal structure and (b) magnetic behavior for 1.
○ Inorg. Chem.,
2018, 57, 2834-2842.
 Water dispersible metal complex nanoparticles were synthesized using the polyethylene glycol (PEG) coating method. The χmT values at room temperature for the NPs 1-3 were calculated using the Evans method, and the proton relaxation times, T1 and T2, were measured employing MRI. Both relaxation times were observed to decrease with increasing χmT value.
Water dispersible metal complex nanoparticles were synthesized using the polyethylene glycol (PEG) coating method. The χmT values at room temperature for the NPs 1-3 were calculated using the Evans method, and the proton relaxation times, T1 and T2, were measured employing MRI. Both relaxation times were observed to decrease with increasing χmT value.

(a) Synthesis scheme for preparing metal complex nanoparticles and (b) correlation between T2 relaxation time and χmT value.
○ Chem. lett.,
2018, 47, 598-600.
 π-π stacking between GO and Benzene sulfonic acid (BS), Naphthalene sulfonic acid (NS), Pyrene sulfonic acid (PS), and Naphthalene disulfonic acid (ND) has resulted in the formation of respective GO-BS, GO-NS, GO-PS and GO-ND hybrid materials. The proton conductivities of these materials follow the trend as GO-NS ˃ GO-BS ˃ GO-PS ˃ GO ˃ GO-ND. GO-NS possessing the highest interlayer distance exhibits the optimum proton conductivity. Evidently, GO-sulfonic acid hybrids reveal excellent superionic conductivity.
π-π stacking between GO and Benzene sulfonic acid (BS), Naphthalene sulfonic acid (NS), Pyrene sulfonic acid (PS), and Naphthalene disulfonic acid (ND) has resulted in the formation of respective GO-BS, GO-NS, GO-PS and GO-ND hybrid materials. The proton conductivities of these materials follow the trend as GO-NS ˃ GO-BS ˃ GO-PS ˃ GO ˃ GO-ND. GO-NS possessing the highest interlayer distance exhibits the optimum proton conductivity. Evidently, GO-sulfonic acid hybrids reveal excellent superionic conductivity.

(a) Sulfonic acid derivative in GO interlayer (b)Proton conductivity for humidity dependence
○ Chemelectrochem,
2018, 5, 238-241.
 Tuneable pressure effects associated with changing interlayer distances
A morphology transformation of hybrid liposomes was shown to occur from spherical vesicles to tubular micelles when increasing the ratio of the metal complex lipid present. Phase transition temperatures increased while viscosities decreased, indicating that the hybrids exhibit stronger interaction between heads but weaker interaction between alkyl chains than occurs in pristine liposomes.
Tuneable pressure effects associated with changing interlayer distances
A morphology transformation of hybrid liposomes was shown to occur from spherical vesicles to tubular micelles when increasing the ratio of the metal complex lipid present. Phase transition temperatures increased while viscosities decreased, indicating that the hybrids exhibit stronger interaction between heads but weaker interaction between alkyl chains than occurs in pristine liposomes.

○ Chem. Commun.,
2017 , 53, 13249-13252.
.
 Tuneable pressure effects associated with changing interlayer distances
in two-dimensional graphene oxide (GO)/reduced GO (rGO) layers are demonstrated
through monitoring the changes in the spin-crossover (SCO) temperature
(T1/2) of [Fe(Htrz)2(trz)](BF4) nanoparticles (NPs) incorporated in the interlayer spaces of the GO/rGO
layers. The interlayer separation along the GO to GO/rGO-NP composites
to rGO series decreases smoothly from 9.00 Å (for GO) to 3.50 Å (for rGO)
as the temperature employed for the thermal reduction treatments of the
GO-NP composites is increased. At the same time, T1/2 increases from 351 K to 362 K along the series. This T1/2 increment of 11 K corresponds to that observed for pristine [Fe(Htrz)2(trz)](BF4) NPs under a hydrostatic pressure of 38 MPa. The influence of
the stacked layer structures on the pseudo-pressure effects has been further
probed by investigating the differences in T1/2 for [Fe(Htrz)2(trz)](BF4) that is present in the composite as larger bulk particles rather than
as NPs.
Tuneable pressure effects associated with changing interlayer distances
in two-dimensional graphene oxide (GO)/reduced GO (rGO) layers are demonstrated
through monitoring the changes in the spin-crossover (SCO) temperature
(T1/2) of [Fe(Htrz)2(trz)](BF4) nanoparticles (NPs) incorporated in the interlayer spaces of the GO/rGO
layers. The interlayer separation along the GO to GO/rGO-NP composites
to rGO series decreases smoothly from 9.00 Å (for GO) to 3.50 Å (for rGO)
as the temperature employed for the thermal reduction treatments of the
GO-NP composites is increased. At the same time, T1/2 increases from 351 K to 362 K along the series. This T1/2 increment of 11 K corresponds to that observed for pristine [Fe(Htrz)2(trz)](BF4) NPs under a hydrostatic pressure of 38 MPa. The influence of
the stacked layer structures on the pseudo-pressure effects has been further
probed by investigating the differences in T1/2 for [Fe(Htrz)2(trz)](BF4) that is present in the composite as larger bulk particles rather than
as NPs.

(a) Schematic illustration of the tunable pressure effects caused by the transformation of GO to rGO. (b) between the interlayer distances and the estimated pseudo-pressure values
○ Sci. Rep.,
2017 , 7, 12159.
.
 Zero in-plane thermal expansion (TE) in a two-dimensional (2D) coordination polymer is demonstrated. The combination of components that expand and those that shrink into zigzag layers results in no net area change in the 2D materials with temperature. Single crystals of [Mn(salen)]2[Mn(N)(CN)4(guest)] (salen = N,N′-ethylenebis (salicylideneaminato), guest = MeOH and MeCN) were prepared, and variable-temperature single-crystal X-ray structural analyses demonstrated that these compounds exhibited both anisotropic positive and negative thermal expansion depending on the guest species. The TE behavior results from distortions of the octahedral coordination geometry of [Mn(salen)]+ units in the zigzag layers. When both guests MeOH and MeCN were incorporated into one material, [Mn(salen)]2[Mn(N)(CN)4(MeOH)0.25(MeCN)0.75], zero in-plane TE resulted in a range of temperature between 380 and 440 K.
Zero in-plane thermal expansion (TE) in a two-dimensional (2D) coordination polymer is demonstrated. The combination of components that expand and those that shrink into zigzag layers results in no net area change in the 2D materials with temperature. Single crystals of [Mn(salen)]2[Mn(N)(CN)4(guest)] (salen = N,N′-ethylenebis (salicylideneaminato), guest = MeOH and MeCN) were prepared, and variable-temperature single-crystal X-ray structural analyses demonstrated that these compounds exhibited both anisotropic positive and negative thermal expansion depending on the guest species. The TE behavior results from distortions of the octahedral coordination geometry of [Mn(salen)]+ units in the zigzag layers. When both guests MeOH and MeCN were incorporated into one material, [Mn(salen)]2[Mn(N)(CN)4(MeOH)0.25(MeCN)0.75], zero in-plane TE resulted in a range of temperature between 380 and 440 K.

○ Inorg. Chem.,
2017 , 56, 6225-6233.
.
 Two ferromagnetic coupled multinuclear Ni(II) clusters, [Ni4L14(μ3-OMe)4(CH3OH)4] (1) and [Ni7(HL2)6(μ3-OMe)6]Cl2 (2) were synthesized, and their structures and magnetic behaviors were investigated in detail. 1 has a [Ni4O4] cubane structure while 2 has a wheel-like [Ni@Ni6] arrangement. The temperature-dependent magnetic susceptibilities for 1 and 2 are in accord with the presence of dominating intra-cluster ferromagnetic interactions between Ni(II) ions. The best fits for their behaviors gave the following parameters: J1 = 11.06 cm−1, J2 = 1.43 cm−1, g = 2.29 for 1, and J1 = 6.87 cm−1, J2 = −3.41 cm−1, g = 2.24 for 2.
Two ferromagnetic coupled multinuclear Ni(II) clusters, [Ni4L14(μ3-OMe)4(CH3OH)4] (1) and [Ni7(HL2)6(μ3-OMe)6]Cl2 (2) were synthesized, and their structures and magnetic behaviors were investigated in detail. 1 has a [Ni4O4] cubane structure while 2 has a wheel-like [Ni@Ni6] arrangement. The temperature-dependent magnetic susceptibilities for 1 and 2 are in accord with the presence of dominating intra-cluster ferromagnetic interactions between Ni(II) ions. The best fits for their behaviors gave the following parameters: J1 = 11.06 cm−1, J2 = 1.43 cm−1, g = 2.29 for 1, and J1 = 6.87 cm−1, J2 = −3.41 cm−1, g = 2.24 for 2.

Crystal structures and magnetic behaviors of (a) cubane-type and (b) wheel-type multinuclear nickel(II) clusters.
○ Dalton trans.,
2017 , 46, 8555-8561.
.
 Supramolecular lipophilic complex cations displaying cobalt(II) SCO properties combined with lipid counter ions have been constructed. The influence of odd (n = 15, 17 and 19)/even (n = 16, 18 and 20) long alkyl chains on the formation of wire and rolled sheet arrangements respectively was documented. However, in the case of the alkyl chains with lengths corresponding to n = 0-14, no supramolecular architectures were observed. Overall, this study reveals the supramolecular structural diversity that ionic lipid self-assembled molecules of the present type can display depending on the length of the alkyl chain in the cobalt() complex investigated.
Supramolecular lipophilic complex cations displaying cobalt(II) SCO properties combined with lipid counter ions have been constructed. The influence of odd (n = 15, 17 and 19)/even (n = 16, 18 and 20) long alkyl chains on the formation of wire and rolled sheet arrangements respectively was documented. However, in the case of the alkyl chains with lengths corresponding to n = 0-14, no supramolecular architectures were observed. Overall, this study reveals the supramolecular structural diversity that ionic lipid self-assembled molecules of the present type can display depending on the length of the alkyl chain in the cobalt() complex investigated.

TEM images of (a) [Co(C15-terpy)2](C12-Glu)2 and (b) [Co(C16-terpy)2](C12-Glu)2
○ Chem. Commun.,
2017 , 53, 4685-4687.
.
 Slight changes in the coordination structure of the manganese(V)-nitrido anionic complex, [MnV(N)(CN)4]2−, induced by using a “lipid package” approach markedly made an impact
on the corresponding redox potentials. The single crystals of four lipid
assemblies, [dabco-(CH2)n−1-CH3]2[Mn(N)(CN)4(H2O)]·xH2O (n = 15, 16, 17 and 18; dabco = 1,4-diazabicyclo[2,2,2]octane),
were synthesized and solid-state cyclic voltammetric studies demonstrated
that the [MnV(N)(CN)4]2− anions with smaller “cross” NC–Mn–CN bond angles
exhibit higher redox potentials. The observed trend reflects the energy
change associated with the structural transformation from [MnV(N)(CN)4]2−
to [MnVI(N)(CN)4]2− and is supported by the results of DFT calculations.
The NC–Mn–CN bond angles in the flexible [Mn(N)(CN)4]2− structure exhibit
excellent correlation with the redox potentials of the complexes in the
solid state.
Slight changes in the coordination structure of the manganese(V)-nitrido anionic complex, [MnV(N)(CN)4]2−, induced by using a “lipid package” approach markedly made an impact
on the corresponding redox potentials. The single crystals of four lipid
assemblies, [dabco-(CH2)n−1-CH3]2[Mn(N)(CN)4(H2O)]·xH2O (n = 15, 16, 17 and 18; dabco = 1,4-diazabicyclo[2,2,2]octane),
were synthesized and solid-state cyclic voltammetric studies demonstrated
that the [MnV(N)(CN)4]2− anions with smaller “cross” NC–Mn–CN bond angles
exhibit higher redox potentials. The observed trend reflects the energy
change associated with the structural transformation from [MnV(N)(CN)4]2−
to [MnVI(N)(CN)4]2− and is supported by the results of DFT calculations.
The NC–Mn–CN bond angles in the flexible [Mn(N)(CN)4]2− structure exhibit
excellent correlation with the redox potentials of the complexes in the
solid state.

(a) Crystal structures of the metal complex lipids and (b) Correlation between coordination bond angles and redox potentials.
○ Dalton Trans.,
2017, 46, 3749-3754.
 Cobalt(II) complexes of type [Co(R-C6-terpy)2](BF4)2 (R = A (1) and T (2)) and a co-crystal of type [Co(A-C6-terpy)1.5(T-C6-terpy)0.5](BF4)2 (3) were prepared. We have investigated assembly arrangement of the compounds. A 3-D network structure is present in 1 that incorporates 1-D chains, while 2 adopts a 2-D stacked structure constructed from ladder-type assemblies. For 3, “dimer-rings” consisting of [Co(A-C6-terpy)2]2 and [Co(A-C6-terpy)(T-C6-terpy)]2 units are generated via base-pairing between A and T. It is noteworthy that 3 displays the first crystal structure of a heteroleptic cobalt(II) complex of [Co(A-C6-terpy)(T-C6-terpy)](BF4)2.
Cobalt(II) complexes of type [Co(R-C6-terpy)2](BF4)2 (R = A (1) and T (2)) and a co-crystal of type [Co(A-C6-terpy)1.5(T-C6-terpy)0.5](BF4)2 (3) were prepared. We have investigated assembly arrangement of the compounds. A 3-D network structure is present in 1 that incorporates 1-D chains, while 2 adopts a 2-D stacked structure constructed from ladder-type assemblies. For 3, “dimer-rings” consisting of [Co(A-C6-terpy)2]2 and [Co(A-C6-terpy)(T-C6-terpy)]2 units are generated via base-pairing between A and T. It is noteworthy that 3 displays the first crystal structure of a heteroleptic cobalt(II) complex of [Co(A-C6-terpy)(T-C6-terpy)](BF4)2.

(a) Single crystal structures and (b) temperature dependence of magnetic susceptibilities.
○ Eur. J. Inorg. Chem.,
2016, 23, 7232-7237.
 Insertion of 3-hydroxypropanesulfonicacid (HPS) in the graphene oxide
(GO) interlayer results in high proton conductivity (10-2-10-1 Scm-1),
owing to an improvement in oxygen content, interlayer distance and water
absorbing capacity. This result indicates that hydroxyalkylsulfonicacids
can be perfect guest molecules for improving the proton conductivity of
carbon materials.
Insertion of 3-hydroxypropanesulfonicacid (HPS) in the graphene oxide
(GO) interlayer results in high proton conductivity (10-2-10-1 Scm-1),
owing to an improvement in oxygen content, interlayer distance and water
absorbing capacity. This result indicates that hydroxyalkylsulfonicacids
can be perfect guest molecules for improving the proton conductivity of
carbon materials.

(a) Single crystal structures and (b) temperature dependence of magnetic susceptibilities.
○ Chem. Asian J.,
2017, 12, 194-197.
 Chiral and racemic iron(II) complexes, [Fe((R)-L)2(NCS)2] (1), [Fe((S)-L)2(NCS)2] (2) and [Fe((R,S)-L)2(NCS)2] (3) (L = α-methyl-N-(2-pyridinylmethylene)-cyclohexanemethanamine) were synthesized. All complexes exhibited spin-crossover (SCO) behaviors. Complexes 1 and 2 showed similar gradual SCO behavior and complex 3 more abrupt SCO behavior. From Mössbauer spectrometry, increasing chirality causes an increasing contribution of LS state.
Chiral and racemic iron(II) complexes, [Fe((R)-L)2(NCS)2] (1), [Fe((S)-L)2(NCS)2] (2) and [Fe((R,S)-L)2(NCS)2] (3) (L = α-methyl-N-(2-pyridinylmethylene)-cyclohexanemethanamine) were synthesized. All complexes exhibited spin-crossover (SCO) behaviors. Complexes 1 and 2 showed similar gradual SCO behavior and complex 3 more abrupt SCO behavior. From Mössbauer spectrometry, increasing chirality causes an increasing contribution of LS state.

(a) Single crystal structures and (b) temperature dependence of magnetic susceptibilities.
○ Eur. J. Inorg. Chem.,
2017, 1049-1053.

○ Dalton Trans.
2016, 45, 16784-16788.

○ Chem. Asian J.
2016, 11, 2322-2327.



